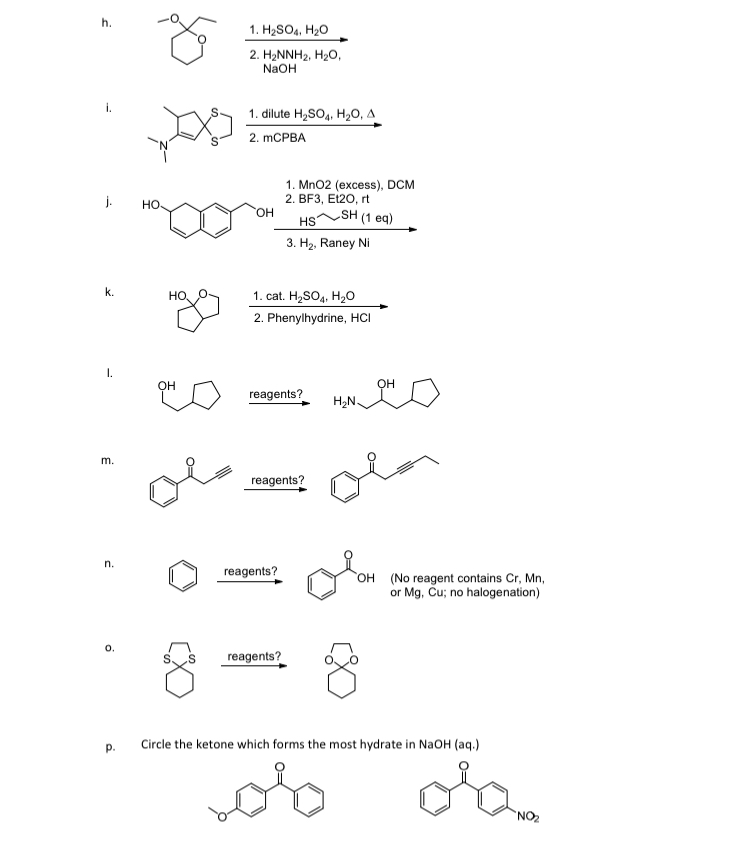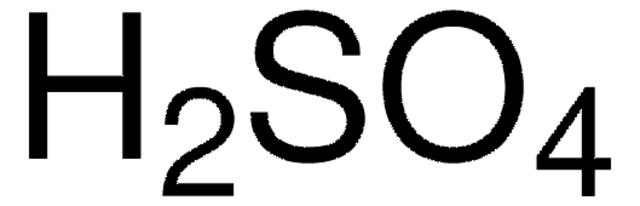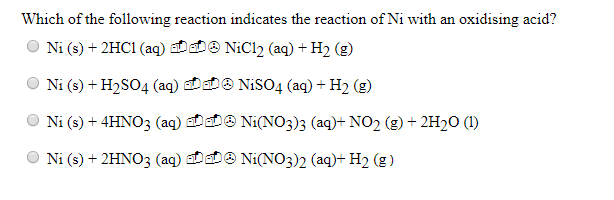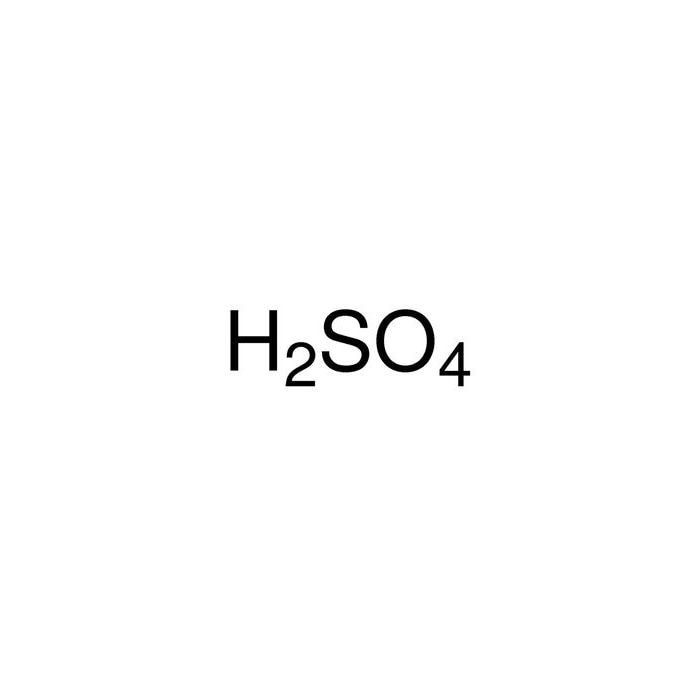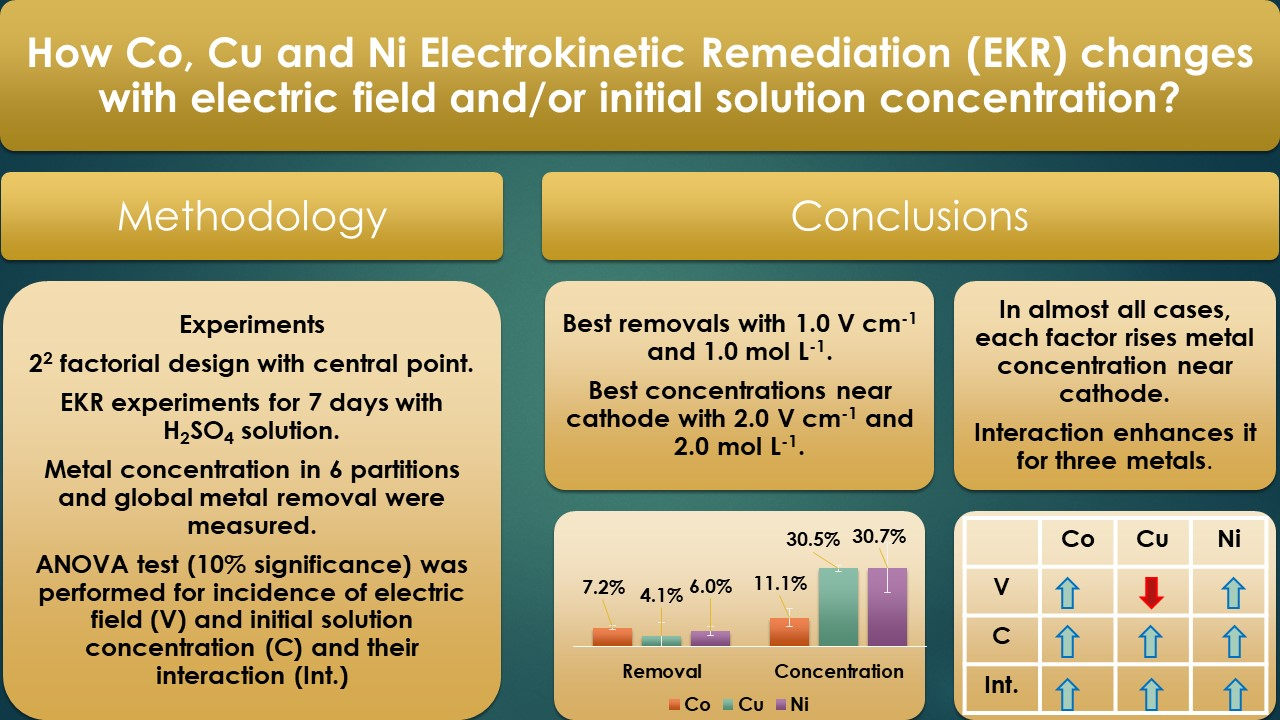
Processes | Free Full-Text | Incidence of Electric Field and Sulfuric Acid Concentration in Electrokinetic Remediation of Cobalt, Copper, and Nickel in Fresh Copper Mine Tailings

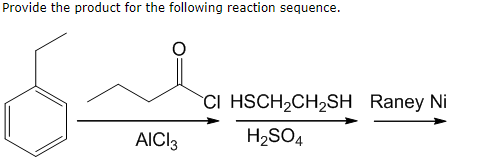
Synergistic Recovery of Valuable Metals from Spent Nickel–Metal Hydride Batteries and Lithium-Ion Batteries | ACS Sustainable Chemistry & Engineering

Comparison between experimental and calculated I-E curves. Cu-Ni in 0.5 M H2SO4 + 10 −4 M cysteine: cathodic scan (cf. Fig. 2).

Leaching Kinetics of Mo, Ni, and Al Oxides from Spent Nickel–Molybdenum Hydrodesulfurization Catalyst in H2SO4 Solution | SpringerLink
![SOLVED: Fe(OH)3, Co(OH)3, Ni(OH)2, MnO2 REACTION WITH [H2SO4, H2O2] HOW TO GET Fe3+ Co2+ Ni2+ Mn2+ JUST WRITE NET IONIC EQUATION FOR 4 OF THEM SOLVED: Fe(OH)3, Co(OH)3, Ni(OH)2, MnO2 REACTION WITH [H2SO4, H2O2] HOW TO GET Fe3+ Co2+ Ni2+ Mn2+ JUST WRITE NET IONIC EQUATION FOR 4 OF THEM](https://cdn.numerade.com/ask_previews/8be369ab-e2f0-4054-a35e-d3e0c372f22f_large.jpg)
SOLVED: Fe(OH)3, Co(OH)3, Ni(OH)2, MnO2 REACTION WITH [H2SO4, H2O2] HOW TO GET Fe3+ Co2+ Ni2+ Mn2+ JUST WRITE NET IONIC EQUATION FOR 4 OF THEM
Effect of H2SO4 concentration on Ni leaching. Conditions: (a) 6 vol.%... | Download Scientific Diagram

a LSVs for the HER on the bare Ni foam (a) and NiBTC/Ni foam (b) in... | Download Scientific Diagram
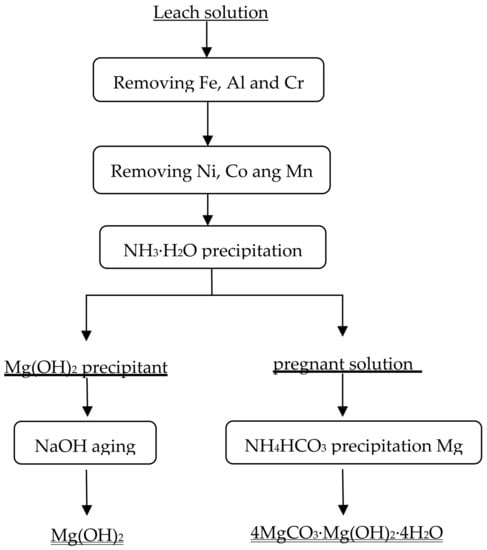
Minerals | Free Full-Text | Recovery of Mg from H2SO4 Leaching Solution of Serpentine to Precipitation of High-Purity Mg(OH)2 and 4MgCO3·Mg(OH)2·4H2O

Kinetics of nickel leaching from low-nickel matte in sulfuric acid solution under atmospheric pressure - ScienceDirect

How to balance Ni+H2SO4=Ni2(SO4)3+H2|Chemical equation Ni+H2SO4=Ni2(SO4)3+H2| Ni+H2SO4=Ni2(SO4)3+H2 - YouTube

Ni+H2SO4=H2+Ni2(SO4)3 Balanced Equation||Nickel+Sulphuric acid=Hydrogen+Nickel sulphate Balanced Equ - YouTube